Abstract
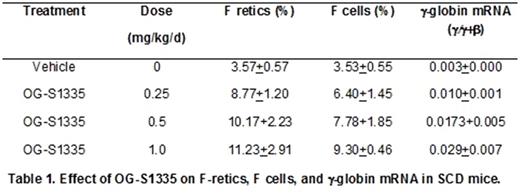
Increased levels of fetal hemoglobin (HbF) lessen the severity of symptoms and increase the life span of patients with sickle cell disease (SCD). New and more effective strategies to increase HbF are needed because the current standard of care, hydroxyurea, is not effective in a significant proportion of patients. Although BM transplantation, gene therapy, and gene editing technologies offer the promise of a cure, it is unlikely that these methodologies can be implemented to treat the large numbers of patients projected worldwide, including those in developing nations that could most easily be treated with an effective, orally administered drug therapy that increased HbF. The lysine-specific demethylase 1A (KDM1A, also referred to as LSD1), is a component of co-repressor complexes that repress γ-globin gene expression and a therapeutic target for HbF reactivation (Shi et al, Nat Med 19:291, 2013). Our laboratory has shown that subcutaneous administration of RN-1, a pharmacological inhibitor of LSD1, increased γ-globin expression in SCD transgenic mice (Rivers et al, Exp Hematol 43:546, 2015; Cui et al, Blood 126:386, 2015) and in baboons (Rivers et al, Haematol 101:688, 2016), widely acknowledged as the best animal model to test the activity of HbF-inducing drugs due to conservation of structure and developmental regulation of the β-globin locus among simian primates. The objective of this investigation was to test whether oral administration of a new LSD1 inhibitor, OG-S1335, could increase HbF in SCD transgenic mice and baboons. Oral administration of varying doses (0.25, 0.5, 1.0mg/kg/d; 4d) of OG-S1335 to SCD mice (3 mice per dose) increased γ-globin expression and the percentage of circulating erythrocytes and reticulocytes positive for HbF (F cells and F retics; Table 1). In baboons, dose escalation was initially performed by administering the drug to a single animal in cycles of 3 days, followed by 1-2 weeks of observation to measure effects on blood parameters. Increased levels of F retics were initially observed at a dose of 100μg/kg/d and subsequently 4 additional baboons were treated with OG-S1335 (by oral gavage) at a dose of either 100 or 200μg/kg/d (7 separate experiments in total). The data presented are in the mean+SD format, with "post-dose" indicating the time point when the highest variation from baseline levels was reported. Increased F retics (pre=8.4+8.9%, post=43.0+27.9%; p<0.01), γ-globin chain synthesis (pre=0.05+0.05 γ/γ+β, post=0.28+0.18 γ/γ+β; p<0.01), and γ-globin mRNA (pre=0.02 +0.02 γ/γ+β, post=0.20+0.11 γ/γ+β) were observed with maximal levels achieved 7 days after administration of the first dose (d8). A decrease in the absolute number of reticulocytes (pre=72.8+17.8 X104/μL, post=35.4+17.8 X 104/μL; p<0.05; nadir on d6-8), neutrophils (pre=4634+994/μL, post=2655+1257/μL; p<0.02; nadir on d13-17), and platelets (pre=406+86 X103/μL, post=168+98 X103/μL; p<0.01; nadir on d8) was also reported, together with increased monocytes (pre=200+68/μL, post=541+213/μL; p<0.01; peak on d10-15). A negative correlation between decreased platelet counts and F retics (r= -0.55), γ-globin chain synthesis (r= -0.92), and γ-globin mRNA (r= -0.80) suggests that variability in drug exposure in addition to possible genetic factors regulating γ-globin expression may be responsible for the observed variability between animals. Additional experiments were performed in two phlebotomized anemic baboons to test the effect of the drug in a model with increased relevance to sickle cell disease. OG-S1335 (100μg/kg/d; 3d) induced high levels of F retics (PA8695, pre-dose=24%, post=66.8%; PA8698: pre-dose=13%, post=93.6%), γ-globin chain synthesis (PA8695: pre-dose=0.07 γ/γ+β, post=0.20 γ/γ+β; PA8698: pre-dose=0.02 γ/γ+β, post=0.44 γ/γ+β) and γ-globin mRNA (PA8695: pre-dose=0.06 γ/γ+β, post=0.18 γ/γ+β; PA8698: pre-dose=0.03 γ/γ+β, post=0.33 γ/γ+β) in each animal. We conclude that oral administration of the LSD1 inhibitor OG-S1335 significantly increased F retics, γ-globin chain synthesis, and γ-globin mRNA in SCD mice and in baboons, supporting further efforts toward the development of this drug for SCD therapy.
Ciceri: Oryzon Genomics SA: Employment. Cavalcanti: Oryzon Genomics SA: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal